
HL Paper 3
Biological pigments include a variety of chemical structures with diverse functions.
The graph shows the conversion of hemoglobin to oxyhemoglobin.
Hb(aq) + 4O2(g) Hb(O2)4(aq)
The partial pressure of oxygen gas, p(O2) is proportional to its concentration.
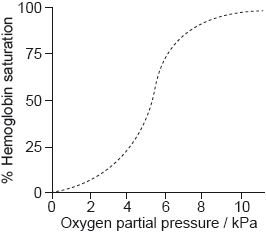
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
Markscheme
binding of O2 «to one active site» affects shape of Hb/other active sites
OR
binding of one O2 «molecule» affects binding of other O2 «molecules»
increasing affinity of Hb to O2
OR
enhanced binding of «further» O2 «molecules»
OR
cooperative binding
[2 marks]
sketching right shift of curve on graph
[1 mark]
decreases «oxygen saturation»
Accept “hemoglobin binds to O2 with less affinity".
[1 mark]
Examiners report
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Methadone is used to treat heroin addiction. 1H NMR spectroscopy can be used to study its structure.
Predict the number of different hydrogen environments in the molecule ignoring the benzene rings.
Predict the chemical shift and the splitting pattern seen for the hydrogens on the carbon atom circled in the diagram. Use section 27 of the data booklet.
Markscheme
6
[1 mark]
Chemical shift:
2.2–2.7 «ppm»
Splitting pattern:
quartet/q
[2 marks]
Examiners report
Ibuprofen and paracetamol are mild analgesics. One of the IR spectra below belongs to ibuprofen and the other to paracetamol. The structures of both compounds are given in section 37 of the data booklet.
Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
Describe how mild analgesics function.
Markscheme
C=O
Accept “carbonyl”.
X (must be identified) AND
Any two of:
For X:
N–H «absorption» AND at 3300 – 3500 «cm–1» ✔
O–H «absorption» in phenol AND at 3200 – 3600 «cm–1» ✔
absence of OH «absorption» in carboxylic acid AND 2500 – 3000 «cm–1»
Accept any specific wavenumber in the range 3300–3380 «cm–1» for M1.
Accept any specific wavenumber in the range 3100–3200 «cm–1».
Award [1 max] if Y is incorrectly identified for paracetamol but if a correct reason/reasons is/are given for the bond absorption(s).
[Max 2 Marks]
prevents/interferes with the production of prostaglandins
OR
prevents/interferes with the production of substances responsible for inflammation/pain/fever at the site of injury/source of pain
Examiners report
Physical properties of elements vary according to atomic number. Sections 6 to 9 of the data
booklet list some of these properties.
Melting points and boiling points of elements 1 to 95
Deduce, giving a reason, the group of elements in the periodic table most likely to undergo sublimation.
Describe the density trend across periods 4 and 5 of the periodic table.
Suggest, with a reason, whether the lanthanoids or actinoids of the f-block would have the higher density.
Compare the ease of oxidation of s-block and d-block metals to their melting points and densities. Use section 25 of the data booklet.
Sketch how the first ionization energies of elements vary with their atomic radius.
Markscheme
group 18/noble gases [✔]
smallest difference between melting and boiling points
OR
weakest intermolecular forces «in that period» [✔]
Note: Accept “group 17/halogens”.
density increases «to a maximum in the transition elements» AND then decreases [✔]
actinoids AND density increases down all groups «due to large increase in atomic mass for small increase in atomic volume»
OR
actinoids AND «much» greater atomic mass with similar type of bonding
OR
actinoids AND density «of actinoids» atomic number 90 to 95 is greater than corresponding lanthanoids [✔]
Note: Accept “actinoids AND on graph actinoids have «much» greater density than lanthanoids”.
Alternative 1:
«metals with» low densities oxidize easier [✔]
«metals with» low melting points oxidize easier [✔]
Alternative 2:
in s-block «metals with» high densities oxidize easier
OR
in s-block «metals with» low melting points oxidize easier [✔]
in d-block «metals with» low densities oxidize easier
OR
in d-block «metals with» low melting points oxidize easier [✔]
Note: Award [1 max] for “s-block metals more easily oxidized” OR “s-block metals have lower melting points” OR “s-block metals have lower densities”.
Accept “have greater activity” for “oxidize easier”.
[✔]
Note: Accept any negative sloping line.
Do not award mark if line touches either axis.
Examiners report
Most candidates correctly identified the group of elements most likely to undergo sublimation but did not score for the reason as they referred to low melting and boiling points, rather than the smallest difference between these temperatures. There were several G2 comments that “the group of elements” was a confusing requirement as elements could be grouped in many ways, including for instance, from B to Ne. The Chemistry Guide clearly states that a group on the periodic table refers to a vertical column of elements. A few complaints were received about the inclusion of a question on sublimation, but the question was designed to make candidates think, and did not require knowledge of phase diagrams.
Required candidates to consider density trends. Most candidates correctly described trends across periods 4 and 5 but had difficulty predicting and explaining whether lanthanoids or actinoids would have the higher density. Many said that actinoids would have higher density because they have more protons and neutrons / greater atomic number / greater mass with no further detail about having similar bonding and hence similar volume. Some G2 comments complained about the inclusion of lanthanoids and actinoids in this question. However, the Chemistry Guide clearly states that these terms should be known.
Most candidates scored at least 1 mark for comparing the s-block and d-block metals and most drew a line with a negative slope.
Although many here failed to score because the line crossed or touched one of the axes. A few sketched a graph reminiscent of first ionization energy against atomic number.
Bromine and methanoic acid react in aqueous solution.
Br2 (aq) + HCOOH (aq) → 2Br− (aq) + 2H+ (aq) + CO2 (g)
The reaction was monitored by measuring the volume of carbon dioxide produced as time
progressed.
Determine from the graph the rate of reaction at 20 s, in cm3 s−1, showing your working.
Outline, with a reason, another property that could be monitored to measure the rate of this reaction.
Describe one systematic error associated with the use of the gas syringe, and how the error affects the calculated rate.
Identify one error associated with the use of an accurate stopwatch.
Markscheme
tangent drawn to curve at t = 20 s [✔]
slope/gradient calculation [✔]
0.35 «cm3 s–1» [✔]
Note: Accept values in the range 0.32–0.42 «cm3 s–1»
ALTERNATIVE 1
colour [✔]
Br2 /reactant is coloured «Br– (aq) is not» [✔]
ALTERNATIVE 2
conductivity [✔]
greater/increased concentration of ions in products [✔]
Note: Do not accept “changes in temperature” or “number of bubbles”.
ALTERNATIVE 3
mass/pressure [✔]
gas is evolved/produced [✔]
Note: Do not accept “mass of products is less than mass of reactants”.
ALTERNATIVE 4
pH [✔]
methanoic acid is weak AND HBr is strong
OR
increase in [H+] [✔]
ALTERNATIVE 1
gas may leak/be lost/escape
OR
plunger may stick/friction «so pressure is greater than atmospheric pressure»
OR
syringe may be tilted «up» so plunger moves less «with gravity acting on plunger»
OR
CO2 dissolved in water [✔]
calculated rate lower [✔]
ALTERNATIVE 2
syringe may be tilted «down» so plunger moves more «with gravity acting on plunger»
OR
syringe is held in hand so gets warmer and gas expands [✔]
calculated rate higher [✔]
Note: Calculated rate is lower or higher must be stated for M2.
Do not accept “scale on syringe is inaccurate”, “errors in reading syringe”, or “bubbles in syringe”.
human reaction time/delay «starting/stopping the stopwatch» [✔]
Examiners report
This part proved to be challenging for some candidates whereas other candidates were able to draw a tangent at 20 sec and then calculate the rate. A significant number of candidates calculated the average rate and achieved one mark.
Majority of the candidates stated another property, which could be monitored correctly. The most common error was changes in temperature, which was stated by some candidates.
This part was about the systematic error and was answered correctly, but many candidates failed to state how the error affected the calculated rate. Many candidates confused this with the concept of a random error and identified the uncertainty of reading the syringe, which is incorrect.
This part was well answered by most candidates although some candidates did not read the question clearly and commented on the stopwatch not working properly or not being accurate.
Aspirin is one of the most widely used drugs in the world.
Aspirin was synthesized from 2.65 g of salicylic acid (2-hydroxybenzoic acid) (Mr = 138.13) and 2.51 g of ethanoic anhydride (Mr = 102.10).
Suggest two absorbances, other than the absorbances due to the ring structure and C–H bonds, that would be present in the infrared (IR) spectrum of aspirin.
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
Markscheme
Any two of:
2500–3000 «cm–1» / «absorbance» due to O–H in carboxyl
1700–1750 «cm–1» / «absorbance» due to C=O in carboxyl/ethanoate
1050–1410 «cm–1» / «absorbance» due to C–O bond in carboxyl/ethanoate
Accept “carboxylic acid” for “carboxyl”, “acetate/ester” for “ethanoate”.
Accept specific wavenumber once within indicated range.
Do not award mark if reference is made to an alcohol/ether.
[2 marks]
Any two of:
melting point
mass spectrometry/MS
high-performance liquid chromatography/HPLC
NMR/nuclear magnetic resonance
X-ray crystallography
elemental analysis
Accept “spectroscopy” instead of “spectrometry” where mentioned but not “spectrum”.
Accept “ultraviolet «-visible» spectroscopy/UV/UV-Vis”.
Do not accept “gas chromatography/GC”.
Accept “thin-layer chromatography/TLC” as an alternative to “HPLC”.
[2 marks]
Examiners report
Alloys containing at least 60 % copper reduce the presence of bacteria on their surface.The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to 250.0 cm3 with water before analysis.
Cu (s) + 4HNO3 (aq) → Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
3Zn (s) + 8HNO3 (aq) → 3Zn(NO3)2 (aq) + 2NO (g) + 4H2O (l)
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
You may find the following chart and diagram helpful.
Outline why the initial reaction should be carried out under a fume hood.
Deduce the equation for the relationship between absorbance and concentration.
Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
Outline how a solution of 0.0100 mol dm−3 is obtained from a standard 1.000 mol dm−3 copper(II) sulfate solution, including two essential pieces of glassware you would need.
The original piece of brass weighed 0.200 g. The absorbance was 0.32.
Calculate, showing your working, the percentage of copper by mass in the brass.
Deduce the appropriate number of significant figures for your answer in (e)(i).
Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (e)(i), use 70 % but this is not the correct answer.
Suggest another property of brass that makes it suitable for door handles.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, Na2S2O3 (aq). Copper(I) iodide is a white solid.
4I− (aq) + 2Cu2+ (aq) → 2CuI (s) + I2 (aq)
I2 (aq) + 2S2O32− (aq) → 2I− (aq) + S4O62− (aq)
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
Markscheme
NO2/NO/NOx/HNO3/gas is poisonous/toxic/irritant ✔
Accept formula or name.
Accept “HNO3 is corrosive” OR “poisonous/toxic gases produced”.
Accept “reaction is harmful/hazardous”.
Slope (gradient):
40 ✔
Equation:
absorbance = 40 × concentration
OR
y = 40x ✔
Accept any correct relationship for slope such as .
Award [2] if equation in M2 is correct.
orange is opposite blue «in the colour wheel»
OR
the complementary colour «blue» is seen/transmitted ✔
585–647 «nm would be absorbed» ✔
Accept any value or range within 550–680 «nm» for M2.
dilute 1.00 cm3 «of the standard solution with water» to 100 cm3
OR
dilute sample of standard solution «with water» 100 times ✔
«graduated/volumetric» pipette/pipet ✔
volumetric flask ✔
Accept any 1 : 100 ratio for M1.
Accept “mix 1 cm3 of the standard solution with 99 cm3 of water” for M1.
Do not accept “add 100 cm3 of water to 1.00 cm3 of standard solution” for M1.
Accept “burette/buret” for M2.
Accept “graduated/measuring flask” for M3 but not “graduated/measuring cylinder” or “conical/Erlenmeyer flask”.
concentration of copper = 0.0080 «mol dm–3» ✔
mass of copper in 250.0 cm3 = «0.0080 mol dm–3 × 0.2500 dm3 × 63.55 g mol–1 =» 0.127 «g»
OR
mass of brass in 1 dm3 = «4 × 0.200 g =» 0.800 g AND [Cu2+] = «0.0080 mol dm–3 × 63.55 g mol–1 =» 0.5084 g dm–3 ✔
«% copper in this sample of brass » 64 «%»
OR
«% copper in this sample of brass » 64 «%» ✔
Accept any value in range 0.0075–0.0085 «mol dm–3» for M1.
Accept annotation on graph for M1.
Award [3] for correct final answer.
Accept “65 «%»”.
two ✔
Do not apply ECF from 1(e)(i).
«since it is greater than 60%» it will reduce the presence of bacteria «on door handles» ✔
resistant to corrosion/oxidation/rusting
OR
low friction surface «so ideal for connected moving components» ✔
Accept “hard/durable”, “«high tensile» strength”, “unreactive”, “malleable” or any reference to the appearance/colour of brass (eg “gold-like”, “looks nice” etc.).
Do not accept irrelevant properties, such as “high melting/boiling point”, “non-magnetic”, “good heat/electrical conductor”, “low volatility”, etc.
Do not accept “ductile”.
precipitate/copper(I) iodide/CuI makes colour change difficult to see
OR
release of I2/iodine from starch-I2 complex is slow so titration must be done slowly ✔
Examiners report
Solubility plays an important role in the bioavailability of drugs in the body.
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
Some mild analgesics contain a solid mixture of acidic aspirin and a non-acidic organic chemical of similar polarity to asprin.
Discuss how acid-base properties and the process of solvent extraction can be used to separate aspirin from the mixture.
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
Markscheme
presence of «large» benzene/arene ring AND non-polar/hydrophobic
OR
presence of «large» benzene/arene ring AND cannot form H-bond with water
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND polar/hydrophilic
OR
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND can form H-bonds with water
Accept “phenyl” for “benzene ring”.
Accept "carboxylic acid" for "carboxyl".
Do not accept "alcohol" for "hydroxyl".
[2 marks]
«student’s» sample impure
lattice disrupted/not uniform «due to presence of impurities»
OR
fewer interparticle/intermolecular forces «due to presence of impurities»
Accept converse arguments.
[2 marks]
One similarity:
peak at 2500–3000 «cm–1»/peak due to O–H/hydroxyl in carboxylic acids
OR
peak at 1700–1750 «cm–1»/peak due to C=O/carbonyl
OR
peak at 2850–3090 «cm–1»/peak due to C–H of arene
One difference:
peak at 3200–3600 «cm–1» in salicylic acid/ peak due to O–H in phenol in salicylic acid
OR
«two» peaks at 1700–1750 «cm–1» in aspirin AND one peak «in the same area» in salicylic acid
Accept “peak at 1600 cm–1 for arene/benzene ring” – not in the data booklet.
Accept “2500–3600 cm–1 «overlapping absorptions of two O–H» in salicylic acid”.
Accept “stronger/broader/split peak at 1700–1750 cm–1 in aspirin”.
[2 marks]
dissolve compounds in an organic solvent
add NaOH(aq)/OH–(aq) «to the mixture» to convert aspirin to its water soluble salt
separate the two «immiscible» layers
convert salt «in aqueous layer» back to aspirin by reacting with acid/H+
«evaporate solvents and dry»
Accept organic solvents immiscible with water such as hexane, ethyl ethanoate, butyl acetate.
Accept any other base.
Need explanation for mark.
[3 marks]
«use of» alternative solvents such as supercritical/liquid CO2
OR
use of water «as solvent»
OR
solvent-free reactions «for example, polymerization of propene»
OR
solid-state chemistry
OR
recycle «waste» solvents
OR
catalysis that leads to better/higher yield
OR
reducing number of steps
Do not accept political/regulatory solutions.
“catalysis” not sufficient for mark.
[1 mark]
Examiners report
Polymers have a wide variety of uses but their disposal can be problematic.
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
The infrared (IR) spectrum of polyethene is given.
Suggest how the IR spectrum of polychloroethene would diff er, using section 26 of the data booklet.
Explain how plasticizers affect the properties of plastics.
Suggest why the addition of plasticizers is controversial.
Outline, giving a reason, how addition and condensation polymerization compare with regard to green chemistry.
Draw the full structural formula of the organic functional group formed during the polymerization of the two reactants below.
Markscheme
correct bonding [✔]
Cl atoms all on same side and alternate [✔]
Note: Continuation bonds must be shown.
Award [1 max] if less than or more than four units shown.
Accept a stereo formula with all atoms and bonds shown.
«strong additional» absorption at 600–800 «cm–1» [✔]
Any two of:
embedded/fit between chains of polymers [✔]
prevent chains from forming crystalline regions [✔]
keep polymer strands/chains/molecules separated/apart [✔]
increase space/volume between chains [✔]
weaken intermolecular/dipole-dipole/London/dispersion/instantaneous dipoleinduced dipole/van der Waals/vdW forces «between chains» [✔]
increase flexibility/durability/softness [✔]
make polymers less brittle [✔]
Note: Accept “lowers density/melting point”.
leach into foodstuffs/environment
OR
«unknown» health/environmental consequences [✔]
Note: Accept “plasticizers cannot be recycled”.
addition produces only the polymer «AND more green»
OR
addition has no by/side-product/condensation produces by-product/small molecules/HCl/NH3 «AND less green»
OR
addition has high atom economy/condensation has lower atom economy «AND less green»
OR
condensation polymers «often» more biodegradable than addition polymers «AND more green» [✔]
Note: Accept “if water produced by condensation «AND condensation and addition equally green»”.
Accept for addition “all of reactants change into products”.
[✔]
Note: Continuation bonds must be shown.
Do not accept condensed formula.
Examiners report
Few candidates scored at least one mark although most either scored both or none for this polymer structure. Some did not read that only four monomer units are required.
Almost all candidates received the mark for identifying the correct absorption band for polychloroethene.
This was a well-answered question; with most candidates identifying at least one method plasticizers affect the properties of plastic.
Several candidates wrote vague answers as to why the addition of plasticizers is controversial.
Candidates seemed to have difficulty in comparing addition and condensation polymerisation with regard to green chemistry.
Several candidates struggled to draw the full structural formula of the peptide linkage formed during the polymerisation of the two reactants.
Changes in physiology can impact living creatures.
The graph shows the change in oxygen partial pressure in blood, measured at different pH values.
Explain the effect of changing pH on the percentage saturation of hemoglobin at a given partial pressure of oxygen.
Explain the biomagnification of the pesticide DDT.
Vitamins are organic compounds essential in small amounts.
State the name of one functional group common to all three vitamins shown in section 35 of the data booklet.
Markscheme
as pH decreases, protons/CO2 bind to allosteric sites
OR
as pH decreases, protons/CO2 act as non-competitive inhibitor
OR
active/binding site changes shape ✔
saturation decreases
OR
more oxygen released
OR
affinity to oxygen decreases ✔
accumulates in fat/tissues/living organisms
OR
cannot be metabolized/does not break down «in living organisms»
OR
not excreted / excreted «very» slowly ✔
passes «unchanged» up the food chain
OR
increased concentration as one species feeds on another «up the food chain» ✔
NOTE: Accept “lipids” for “fat”.
hydroxyl ✔
NOTE: Accept “hydroxy” but not “hydroxide”.
Accept “alkenyl”.
Do not accept formula.